A Titration is a common laboratory experimental technique used to determine the concentration of an unknown solution! Because volume measurements play a key role in titration, it is also known as volumetric analysis.
So what is needed to conduct a titration experiment?
1. Buret
3. Pipet (Glass tube) & Erlenmeyer Flask
4. Indicators- Used to identify the end point of filtration
5. Stock solution - Known Solution
All together your lab should look like ours, ready to go!!! (Although we're conducting titration experiments next class, this is how i envision our lab to look like lol)
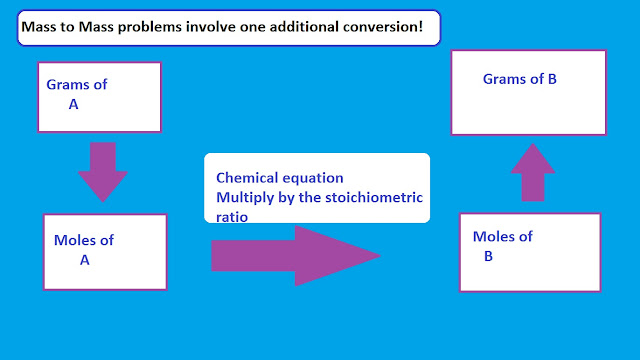
Okay so today in class, we learned how to use the information given to us to determine the concentration of such and such substances:
Dora the ultimate explorer completed a titration of 0.330 M NaOH with 15.00 mL samples of HI of unknown concentration. The data he gathered is below. Determine the concentration of HI.
Next we add up 11.2 mL + 10.9 mL + 11.4 mL = divide by 3 = 11.16666667 mL
*Note we don't have to use 8.9 because it's not a close number to the others
Next we change 11.16 mL into L
11.16 x 1
1000
= .0111L
Next we take our concentration and change that into Moles of HI
0.330 M x .011
Finally, we take the moles we got (0.00363)
and divide it by the litres we initially received (15.00 mL)
*remember we must change mL into units of L!
15.00
1000
= .015 L
Now we have moles and litres, and Concentration is Moles/Litres so all we have to do is divide the 2!
0.00363 mol
.015 L
= .246 M
Fairly straight forward right?
If you want to know more about what Titration is, here is youtube video to watch!
Post by Renren