Re-cap of all that we have learned depicted through this picture:
27.12.10
yet another... Density to Moles
Just when you think all the conversions are over, from moles to atoms to atoms to moles, we still have one more!
But don't worry! It's pretty easy! Today in class we learned about converting from Density to Moles.
Density is a measure of Mass per volume.
D= M/V
It is usually measured in g/L (grams per liter) or g/ml (grams per milliliter)
Lets try a few Questions shall we?
A 2.5 g sample of an unknown metal has a volume of 32 mL. Determine the metal's density.
Since we are given grams and mL, we simply divide 2.5g/32mL to get 0.078g/mL
Remember density can be measured in g/mL!
(DENSITY-MOLES)
How many moles are in a 25.0mL sample of Iron if the density of Iron is 7.87 g/mL?
start with the given density:
7.87g/mL x 25.0 mL = 196.75g x 1 mol/55.8g = 3.53 mol
(GIVEN VOLUME/MOL- FIND DENSITY)
If a gold ring has a volume of 7.50 mL and contains 0.736 mol of Gold determine the density of Gold.
You are given the volume in mL so you must find the mass!
Take 0.736 mol and use it in this equation:
0.736mol x 197.0g/1 mol = 144.992 g
Now since you've figured out grams, the formula for density is d=m/v
You have mass= 144.992g
You have volume= 7.50mL
now divide the two to get
19.3 g/mL
(DENSITY-ATOMS)
Copper has a density of 8.96 g/mL. Determine the number of atoms in copper key that has a volume of 20mL.
8.96 g/ml x 20ml = 179.2 g x 1mol/63.5g = 2.82 mol x 6.02 x 10^23 atoms/1mol
= 1.7 x 10^24atoms
We also learned about the Density of gases!
The Density of gases varies with temperature!
New formula:
Lets try some questions!
Determine the density of Methane (CH4) at STP
Molar mass->16.0g/mol
Molar volume->22.4L/mol
= .714 g/L
An unknown diatomic gas has a density of 1.25 g/L at STP. Determine the Molar mass for this gas then write the chemical formula for it.
1.25 g/L x 22.4 L/1mol
= 28 g/mol
= 28g/mol
2
(You divide by 2 because we are looking for an "unknown diatomic gas")
Once you divide by 2, you get
14 g/mol
What element on the periodic table has a molar mass of 14g?
Nitrogen!
N2!
And that is all we learned :)
Post by REN
Mole to Atom/Molecule conversions
Today we learned how to convert from moles to atoms or from moles to molecules
Pretty basic stuff if I do say so myself!
Determine the number of atoms that are in 1.25 mol of O2
1.25mol x 6.02x10^23 atoms/ 1 mol = (7.53 x 10^23)
But since we're looking for Oxygen gas (diatomic) we times our answer by 2.
= (7.53 x 10^23) x 2
= 1.51 x 10^24 atoms
Determine the number of atoms that are in 0.58 mol of Se
0.58mol x 6.02 x 10^23 atoms/ 1 mol = 3.5 x 10^23 atoms
2. FROM ATOMS TO MOLES
How many moles are present in 3.9 x10^25 Nitrogen atoms?
3.9x10^ 25 Nitrogen atoms x 1 mol/6.02x10^23 atoms= 64.8 moles
A cylinder of Helium contains 4.6 x 10^30 atoms. How many moles of Helium is this?
4.6 x 10^30 atoms x 1 mol/ 6.02 x 10^23 atoms = 7.6 x 10^6 mol
Are you slowly realizing that conversions aren't at all that difficult like this fellow right here?
GOOD lets try another one, but this time even more complicated!
How many water molecules are there in 0.65 mol? How many hydrogen atoms are there? How many oxygen atoms are there?
If your initial reaction looks something like this^..... than follow the steps below!
First step: Do not panic after reading this question! Lets take it STEP BY STEP!
Let us first find how many water molecules is in 0.65 moles
0.65 mol x 6.02 x 10^ 23 molecules / 1 mol
You are trying to find molecules/atoms so put that on top!
Cancel out units of moles and times 0.65 and 6.02 x 10^23 and you will get
3.9 x 10 ^ 23 molecules
How many hydrogen atoms are there?
Remember they are asking for water molecules, and the chemical equation for water is H20
In this equation we see 2 Hydrogen atoms so...
(3.9x10^23atoms) x 2 = 7.8 x 10^23 atoms of Hydrogen
How many oxygen atoms? There is no subscript next to Oxygen so we assume its 1
(3.9x10^23atoms) x 1= 3.9 x 10^23 atoms of oxygen
Yay! You did it!
Post by Ren Flores
Pretty basic stuff if I do say so myself!
- FROM MOLES TO ATOMS:
Determine the number of atoms that are in 1.25 mol of O2
1.25
But since we're looking for Oxygen gas (diatomic) we times our answer by 2.
= (7.53 x 10^23) x 2
= 1.51 x 10^24 atoms
Determine the number of atoms that are in 0.58 mol of Se
0.58
2. FROM ATOMS TO MOLES
How many moles are present in 3.9 x10^25 Nitrogen atoms?
3.9x10^ 25 Nitrogen
A cylinder of Helium contains 4.6 x 10^30 atoms. How many moles of Helium is this?
4.6 x 10^30
Are you slowly realizing that conversions aren't at all that difficult like this fellow right here?
GOOD lets try another one, but this time even more complicated!
How many water molecules are there in 0.65 mol? How many hydrogen atoms are there? How many oxygen atoms are there?
If your initial reaction looks something like this^..... than follow the steps below!
First step: Do not panic after reading this question! Lets take it STEP BY STEP!
Let us first find how many water molecules is in 0.65 moles
0.65 mol x 6.02 x 10^ 23 molecules / 1 mol
You are trying to find molecules/atoms so put that on top!
Cancel out units of moles and times 0.65 and 6.02 x 10^23 and you will get
3.9 x 10 ^ 23 molecules
How many hydrogen atoms are there?
Remember they are asking for water molecules, and the chemical equation for water is H20
In this equation we see 2 Hydrogen atoms so...
(3.9x10^23atoms) x 2 = 7.8 x 10^23 atoms of Hydrogen
How many oxygen atoms? There is no subscript next to Oxygen so we assume its 1
(3.9x10^23atoms) x 1= 3.9 x 10^23 atoms of oxygen
Yay! You did it!
Post by Ren Flores
14.12.10
MOLAR VOLUME!!!
Today we learned about Moles to Volume conversions.... ooooo sounds complicated? Well it's actually not too shabby! (Does anyone use that word anymore?)
Before we start please note:
At a specific pressure and temperature one mole of any gas occupies the same volume!
Note: *at 0 degrees Celsius and 101.3 kilo Pascals, 1 mole : 22.4 Litres
This temperature and pressure is called STP which stands for Standard Temperature Pressure.
22.4 L/mol is the molar volume at STP
Lets try some examples shall we.....
How many liters will 2.5 mol of H2 occupy at STP?
Start with what you're given... which is 2.5 mol
You want to find how many liters are in 2.5 mol of H2!
Your equation will look like this:
2.5mol x 22.4 L/ 1 mol = 56 L
Lets try some more! Isn't this fun
-----> A certain gas is found to occupy 11.6 L at STP. How many moles?
11.6 L x 1 mol / 22.4 L = .518 mol
------> At STP an unknown gas is found to occupy 150mL. How many moles of gas must there be?
First off, we see that the volume is measured in mL. We must change it to liters!
First step: 150mL x 1 L / 1000 mL = 0.150 L
Second step: 0.150 L x 1 mol/ 22.4 L = 0.00670 mol
Remember significant digits! In this case, we round to 3 sig figs!
Getting there? GOOD! :)
alright so let's try one more example and lets see if you get the gist of it all!
A certain amount of Chlorine gas occupies 1.6 L. Find the # of moles present and then determine the mass of chlorine.
So in this specific equation, we are asked to find 2 things. The number of moles present and the mass of chlorine. We shall start off with what we're given!
1.6L x 1 mol / 22.4 L = 0.71 mol
You figured out the number of moles! Now find the mass of chlorine!
0.71 mol x 71 g/ 1 mol = 5.1 g
Dont forget Chlorine is diatomic so the equation on the top would be 2(35.5) which gave us the number 71!
And das it! Good job everyone!

Post by Ren Ren
Before we start please note:
At a specific pressure and temperature one mole of any gas occupies the same volume!
Note: *at 0 degrees Celsius and 101.3 kilo Pascals, 1 mole : 22.4 Litres
This temperature and pressure is called STP which stands for Standard Temperature Pressure.
22.4 L/mol is the molar volume at STP
Lets try some examples shall we.....
How many liters will 2.5 mol of H2 occupy at STP?
Start with what you're given... which is 2.5 mol
You want to find how many liters are in 2.5 mol of H2!
Your equation will look like this:
2.5
Lets try some more! Isn't this fun
-----> A certain gas is found to occupy 11.6 L at STP. How many moles?
11.6
------> At STP an unknown gas is found to occupy 150mL. How many moles of gas must there be?
First off, we see that the volume is measured in mL. We must change it to liters!
First step: 150
Second step: 0.150 L x 1 mol/ 22.4 L = 0.00670 mol
Remember significant digits! In this case, we round to 3 sig figs!
Getting there? GOOD! :)
alright so let's try one more example and lets see if you get the gist of it all!
A certain amount of Chlorine gas occupies 1.6 L. Find the # of moles present and then determine the mass of chlorine.
So in this specific equation, we are asked to find 2 things. The number of moles present and the mass of chlorine. We shall start off with what we're given!
1.6
You figured out the number of moles! Now find the mass of chlorine!
0.71 mol x 71 g/ 1 mol = 5.1 g
Dont forget Chlorine is diatomic so the equation on the top would be 2(35.5) which gave us the number 71!
And das it! Good job everyone!

Post by Ren Ren
6.12.10
MOLAR MASS
Molar mass: The molar mass is the mass, in grams, of one mole of a substance. The molar mass is numerically the same as the formula mass (found in the periodic table) but with units of grams mol-1.
We use grams per mol = g/mol
To calculate the molar masses:
Here are some other examples:
Calculate the molar masses of:
Mg(OH)2
24.3 + 2(16) + 2(1) = 58.3 g/mol
(NH4)2SO4
2(14.0)+8(1.0)+32.1+4(16.0)= 132 g/mol
Now let's try some Molar Conversions...
Mr. Doktor gave us a handy chart we can refer to when we are doing our conversions! Look below:
Now let us try shall we...
oh yeah before I forget, remember to use Avogardo's top secret number when you are trying to find the number of atoms, formula units, or moles! Whats his secret number?
Highlight the bottom space under this to find out....
6.02 x 10²³
Now dont tell anyone and use this number to your advantage.... haha
A sample of Carbon contains 2.47x10²5 atoms. How many moles of carbon is this?
2.47x10²5atoms x 1 mol/ 6.02 x 10²³ atoms = 41.02990033 moles
*Round to sig figs
= 41.0 moles
How many atoms are there in 1.5 mol of Iron?
(If your stuck, start off with what you're given!)
1.5mol x 6.02 x10²³atoms/1 mol= 9.03 x10²³ atoms
... but you only get 1/2 marks because you forgot sig figs! so remember your sig figs and you get....
= 9.0 x 10²³ atoms
12.5 moles of 0² represent how many molecules?
12.5mol x 6.02x10²³molecules/ 1mol = 7.52 x ²4 molecules
Find the mass of 0.159 mol of SiO2
0.159mol x 60.1 g/1mol = 9.5559g
=9.56g
Get the drift? If yes than good for you now go away! If no, then lets do one more with a more detailed explanation. Lets try the a similar example to the last example I did.
Find the mass of 3.66 mol of N²
Write what you're given:
3.66 mol
Now you want to get rid of moles and you want to find the mass. Mass is measured in grams (g) and since N (nitrogen) is diatomic (which you can also depict from the subscript 2 however that 2 should not be on the top right it should be on the bottom...) then you can write your equation like this:
3.66 mol x 28 g / 1 mol
28 grams came from Nitrogens Atomic mass in the periodic table which is 14 but times 2! 14x 2 = 28!
3.66mol x 28g/ 1 mol
Cancel your units....
(3.66) x (28)
and you get ....
102 grams!
Now you know how to convert mole conversions! More coming later!
-Post by Ren Flores
We use grams per mol = g/mol
To calculate the molar masses:
Here are some other examples:
Calculate the molar masses of:
Mg(OH)2
24.3 + 2(16) + 2(1) = 58.3 g/mol
(NH4)2SO4
2(14.0)+8(1.0)+32.1+4(16.0)= 132 g/mol
Now let's try some Molar Conversions...
Mr. Doktor gave us a handy chart we can refer to when we are doing our conversions! Look below:
Now let us try shall we...
oh yeah before I forget, remember to use Avogardo's top secret number when you are trying to find the number of atoms, formula units, or moles! Whats his secret number?
Highlight the bottom space under this to find out....
6.02 x 10²³
Now dont tell anyone and use this number to your advantage.... haha
A sample of Carbon contains 2.47x10²5 atoms. How many moles of carbon is this?
2.47x10²5
*Round to sig figs
= 41.0 moles
How many atoms are there in 1.5 mol of Iron?
(If your stuck, start off with what you're given!)
1.5
... but you only get 1/2 marks because you forgot sig figs! so remember your sig figs and you get....
= 9.0 x 10²³ atoms
12.5 moles of 0² represent how many molecules?
12.5
Find the mass of 0.159 mol of SiO2
0.159
=9.56g
Get the drift? If yes than good for you now go away! If no, then lets do one more with a more detailed explanation. Lets try the a similar example to the last example I did.
Find the mass of 3.66 mol of N²
Write what you're given:
3.66 mol
Now you want to get rid of moles and you want to find the mass. Mass is measured in grams (g) and since N (nitrogen) is diatomic (which you can also depict from the subscript 2 however that 2 should not be on the top right it should be on the bottom...) then you can write your equation like this:
3.66 mol x 28 g / 1 mol
28 grams came from Nitrogens Atomic mass in the periodic table which is 14 but times 2! 14x 2 = 28!
3.66
Cancel your units....
(3.66) x (28)
and you get ....
102 grams!
Now you know how to convert mole conversions! More coming later!
-Post by Ren Flores
16.11.10
HOLY MOLEY
Avogadro's Numbers
-atoms and molecules are extremely small and contains too many to count or weigh individually.
-Amedeo Avogadro proposed that the number of atoms 12.00000g of carbon be equal to a constant (this is equal to 1 mol of carbon)
-this value is also called Avogadro's number and forms the basis of all quantitive chemistry.
Avogadro's Number: 6,020,000,000,000,000,000,000,000 = 6.02x10 to the power of 23
-1.0 mole = 6.0x10 to the power of 23
-1 mole is simply a multiple of things.
*one mole represents a huge number of particles*
eg: 1 mole of blood cells would be more than the total number of blood cells in every human on earth.
EXAMPLE:
- A sample of carbon contains 2.47x10 to the 25 atoms. How many moles of carbon is this?
[ *write down what you are given]
[ *identify what to cancel ]
-atoms and molecules are extremely small and contains too many to count or weigh individually.
-Amedeo Avogadro proposed that the number of atoms 12.00000g of carbon be equal to a constant (this is equal to 1 mol of carbon)
-this value is also called Avogadro's number and forms the basis of all quantitive chemistry.
Avogadro's Number: 6,020,000,000,000,000,000,000,000 = 6.02x10 to the power of 23
-1.0 mole = 6.0x10 to the power of 23
-1 mole is simply a multiple of things.
*one mole represents a huge number of particles*
eg: 1 mole of blood cells would be more than the total number of blood cells in every human on earth.
EXAMPLE:
- A sample of carbon contains 2.47x10 to the 25 atoms. How many moles of carbon is this?
[ *write down what you are given]
[ *identify what to cancel ]
**remember: your significant digits & to always put your units!
Post by the awesome Ivy Gloria
12.11.10
UPDATE
Here's what has happened during the last 3 classes:
..
Materials Used: - Bunsen burner
- Test tube
- Test tube rack
- Test tube clamp
- Weight scale Procedure 1. Fill a test tube about 1 cm with the hydrate
2. Carefully place the test tube on the scale and record the mass of the hydrate and test tube
3.
4. Pick up the test tube with the clamps and carefully hold it in above the Bunsen burner
5. Gently heat the test tube by moving the test tube in and out of the flame for about 5 minutes or until all the water has boiled away
6. Carefully re-weigh the test tube ensuring none of the chemicals inside spill Using extreme caution, connect and light your Bunsen burner. Adjust the gas flow until the flame is about 5 cm tall
We had a....
Hydrate Lab
Discussion: Hydrates are ionic compounds that contain an inorganic salt compound loosely bound to water. The purpose of this experiment is to determine the empirical formula of a hydrate. In this lab we determined the anhydrous (without water) mass of the hydrate. We compared this with the actual mass of water that should have been present.
..
Materials Used: - Bunsen burner
- Test tube
- Test tube rack
- Test tube clamp
- Weight scale Procedure 1. Fill a test tube about 1 cm with the hydrate
2. Carefully place the test tube on the scale and record the mass of the hydrate and test tube
3.
4. Pick up the test tube with the clamps and carefully hold it in above the Bunsen burner
5. Gently heat the test tube by moving the test tube in and out of the flame for about 5 minutes or until all the water has boiled away
6. Carefully re-weigh the test tube ensuring none of the chemicals inside spill Using extreme caution, connect and light your Bunsen burner. Adjust the gas flow until the flame is about 5 cm tall
...
The following class after the Hydrate Lab, we had a test for Unit 2: The Atomic Theory
During today's class (November 12th, 2010), we had special guests (Japanese students) that visited us from Japan. We had very little time to do anything, however, so we went over our test which was fairly o.k.
-Ren Flores
The following class after the Hydrate Lab, we had a test for Unit 2: The Atomic Theory
During today's class (November 12th, 2010), we had special guests (Japanese students) that visited us from Japan. We had very little time to do anything, however, so we went over our test which was fairly o.k.
-Ren Flores
4.11.10
Naming Compounds
Today we learned about Chemical Nomenclature (giving something a name)
Up to this date, the most common system is the IUPAC (International Union of Pure and Applied Chemistry) and this is a system of naming Chemical Compounds such as
Be aware of the differences between an ION and a Compound!
-Almost all are anions (Most polyatomic ion has a negative charge with Ammonium being an exception since it has a positive charge)
Examples: Sulphate, Cyanide, Nitrate are all Complex ions
What is the chemical formula for sodium nitrate?
---------- NaNo3
Ferrous Sulfite? Fe (+2)So3(+2) --> FeSo3
--------------------------------------------------------------
We also learned about HYDRATES:
-Some Compounds can form lattices that bond to water molecules such as:
a) Copper Sulfate
b) Sodium Sulfate
-These crystals contain water inside them which can be released by heating!
To name Hydrates:
1.Write the name of the Chemical formula
2.Add a pre-fix indicating the number of water molecules (mono=1,do=2,tri=3,tetra=4,penta=5,hexa=6,etc)
3.Add hydrate after the prefix
ie. Cu(SO4) • 5 H20 -> Cuppric Sulphate or Copper (II) Sulphate
Li(ClO4) • 3H2O -> Lithium Perchlorate Trihydrate
Note: Even if there is only one atom present of the second non-metal in the compound, always use the prefix “mono”. Here are the pre-fixes used:
Lastly, we learned how to name ACIDS and BASES
-HYDROGEN compounds are ACIDS
-Hydrogen appears first in the formula unless it is part of a polyatomic group such as
CH3COOH---> Polyatomic Acid = Acetic Acid
ie. H2SO4 --> Sulfuric Acid
Hydroxide, or OH- is found in Bases.
-name as binary ionic
ie. NaOH ---> Sodium Hydroxide
Ba(OH)2 ---> Barium Hydroxide
...and there you have it!
-Post by Ren Flores Aka Birthday Boy who posted a Chem post on his birthday.
Up to this date, the most common system is the IUPAC (International Union of Pure and Applied Chemistry) and this is a system of naming Chemical Compounds such as
- Ions
- Binary Ionic
- Polyatomic Ions
- Molecular Compounds
- Hydrates
- Acids/Bases
Be aware of the differences between an ION and a Compound!
What is the difference between an Ion and an atom?
An Ion makes up the electric charge of an atom. It can be a positively (+) charged atom or a negatively (-) charged atom, depending on the number of protons versus electrons.
On the other hand, an atom is the smallest part of an element composed of electrons, protons, and the nucleus.To add onto Multivalent Ions, some elements can form more than 1 ion. The most common ion charge is listed first, on the top right hand corner. Example: Iron has 2 charges +3 and +2, so +3 would be the more common charge and we use it when it is not given to us.
Multivalent Ions:
Classical systems uses latin names of elements and the suffixes (Listed Below)
For example, to represent Iron and Oxide, we would say Ferric Oxide. Note that ending of Ferr, which is the latin name for Iron is -IC, so we know that the charge used is the top one which is the more common/larger charge.
Another example: Plumbic Oxide, which would be Pb2O4 which simplifies to PbO2
Mercuric Fluorode -> HgF2
COMPLEX IONS
-Complex Ions are large groups of atoms that stay together during a Chemical reaction-Almost all are anions (Most polyatomic ion has a negative charge with Ammonium being an exception since it has a positive charge)
Examples: Sulphate, Cyanide, Nitrate are all Complex ions
What is the chemical formula for sodium nitrate?
---------- NaNo3
Ferrous Sulfite? Fe (+2)So3(+2) --> FeSo3
--------------------------------------------------------------
We also learned about HYDRATES:
-Some Compounds can form lattices that bond to water molecules such as:
a) Copper Sulfate
b) Sodium Sulfate
-These crystals contain water inside them which can be released by heating!
To name Hydrates:
1.Write the name of the Chemical formula
2.Add a pre-fix indicating the number of water molecules (mono=1,do=2,tri=3,tetra=4,penta=5,hexa=6,etc)
3.Add hydrate after the prefix
ie. Cu(SO4) • 5 H20 -> Cuppric Sulphate or Copper (II) Sulphate
Li(ClO4) • 3H2O -> Lithium Perchlorate Trihydrate
Note: Even if there is only one atom present of the second non-metal in the compound, always use the prefix “mono”. Here are the pre-fixes used:
- One —-> Mono
- Two —-> Di
- Three —-> Tri
- Four —-> Tetra
- Five —-> Penta
- Six —-> Hexa
- Seven —-> Hepta
- Eight —-> Octa
- Nine —-> Nona
- Ten —-> Deca
Lastly, we learned how to name ACIDS and BASES
-HYDROGEN compounds are ACIDS
-Hydrogen appears first in the formula unless it is part of a polyatomic group such as
CH3COOH---> Polyatomic Acid = Acetic Acid
ie. H2SO4 --> Sulfuric Acid
Hydroxide, or OH- is found in Bases.
-name as binary ionic
ie. NaOH ---> Sodium Hydroxide
Ba(OH)2 ---> Barium Hydroxide
...and there you have it!
-Post by Ren Flores Aka Birthday Boy who posted a Chem post on his birthday.
3.11.10
QUANTUM MECHANICS:
The basis of quantum mechanics came from Neils Bohr’s theory in 1920...
BOHR THEORY
- the electron is a particle that must be in orbital in the atom
QUANTUM THEORY
- the electron is like a cloud of negative energy or a wave
- the electron wave stays in certain orbitals
- orbitals are areas in 3D space where the electrons most probably are (*Note: we can never be 100% sure the electron is exactly in a certain place, since it is said to be a wave of energy)
- the energy of the electron is in its vibrational modes (like notes on a guitar string)
- photons are produced when high energy modes change to lower energy modes (Example: energy from the electron is released as light, aka a photon)
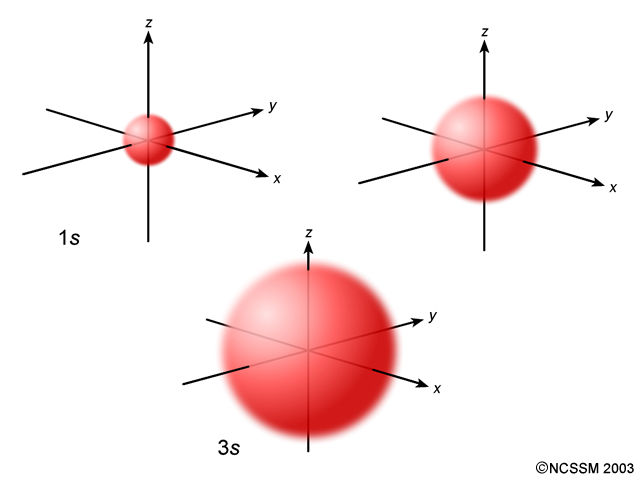
The orbitals contain electrons moving around in that area. The orbitals may overlap eachother; the differences between the orbitals (as we know so far) is that they can be larger spaces, smaller spaces, or situated in different sides.
(*Note: ignore the numbers and words on the pictures. The pictures are just examples of where the orbitals may be situated in the atom.)
S Orbitals
- there is 1 suborbital
- each orbital holds 2 electrons
- total electrons: 2
P Orbitals
- there are 3 suborbitals
- each containing 2 electrons
- total electrons: 6

D Orbitals
- there are 5 suborbitals
- each contains 2 electrons
- total electrons: 10

F Orbitals
- there are 7 suborbitals
- each contains 2 electrons
- total electrons: 14

How the orbitals correspond to the periodic table and elements:

*Note: if an element is under, let's say "2p", it has all the other orbitals filled too, so that would be 1s, 2s, 2p. Geddit?
For Bohr models, we know we draw engery levels and little dots for electrons.
For Lewis Diagrams, we graw the outer shell with dots for electrons.
So how do we express a certain element with it's electrons using this Quantum Mechanic theory?
Example:
Fluorine
1) Find the element on the periodic table
BOHR THEORY
- the electron is a particle that must be in orbital in the atom
QUANTUM THEORY
- the electron is like a cloud of negative energy or a wave
- the electron wave stays in certain orbitals
- orbitals are areas in 3D space where the electrons most probably are (*Note: we can never be 100% sure the electron is exactly in a certain place, since it is said to be a wave of energy)
- the energy of the electron is in its vibrational modes (like notes on a guitar string)
- photons are produced when high energy modes change to lower energy modes (Example: energy from the electron is released as light, aka a photon)
The orbitals contain electrons moving around in that area. The orbitals may overlap eachother; the differences between the orbitals (as we know so far) is that they can be larger spaces, smaller spaces, or situated in different sides.
(*Note: ignore the numbers and words on the pictures. The pictures are just examples of where the orbitals may be situated in the atom.)
S Orbitals
- there is 1 suborbital
- each orbital holds 2 electrons
- total electrons: 2

P Orbitals
- there are 3 suborbitals
- each containing 2 electrons
- total electrons: 6

D Orbitals
- there are 5 suborbitals
- each contains 2 electrons
- total electrons: 10

F Orbitals
- there are 7 suborbitals
- each contains 2 electrons
- total electrons: 14

How the orbitals correspond to the periodic table and elements:

*Note: if an element is under, let's say "2p", it has all the other orbitals filled too, so that would be 1s, 2s, 2p. Geddit?
For Bohr models, we know we draw engery levels and little dots for electrons.
For Lewis Diagrams, we graw the outer shell with dots for electrons.
So how do we express a certain element with it's electrons using this Quantum Mechanic theory?
Example:
Fluorine
1) Find the element on the periodic table
2) Figure out how many orbitals its electrons take up (you can use the previous chart to figure this out, just match the positions!)
So, it's in the 2p section, which means its electrons take up the orbitals 1s, 2s, 2p
3) Next, find out how many electrons it has and figure out how many are in each orbital
Fluorine has 9 electrons
(Since each S orbital can hold 2 electrons...)
(Since each S orbital can hold 2 electrons...)
1s holds a max. of 2 electrons
2s holds a max. of 2 electrons
(Since each P orbital can hold 6 electrons...)
2p holds a max. of 6 electrons
So Fluorine would look like:
1s(2) 2s(2) 2p(5)
(*bracket numbers = the number of electrons in each orbitals)
And you're done!
Try it out using Sodium
Highlight for answer: 1) 1s(2)2s(2)2p(6)
1.11.10
BOHR DIAGRAMS
Bohr Diagrams:
-Atoms are electrically neutral (the number of protons equals the number of electrons)
- Two different models can be used to describe electron configuration
-Energy Level Model
-Bohr Model
- electrons occupy shells which are divided into orbitals
The Bohr diagrams places the number of neutrons and protons in the center and electrons in energy rings around the outside. Each energy ring has a maximum number it can hold
Learned in class:
Ring 1 – 2 electrons
Ring 2 – 8 electrons (octet)
Ring 3 – 8 electrons (octet)
Ring 4 – 18 electrons
----------------------------------------------------------
Ring 5 – 18 electrons
Ring 6 – 32 electrons
Ring 7 – 32 electrons
The electrons in the outer most energy ring are called valence electrons. These electrons are very important because they are involved in chemical reactions. They can be lost or gained so that the element will have 8 electrons in the outer most ring and become stable. The noble gases already have 8 electrons in their outer ring so they are stable and do not react readily
Atom Structure:
http://www.youtube.com/watch?v=hpKhjKrBn9s&annotation_id=annotation_736168&feature=iv
Bohr Diagrams:
http://www.youtube.com/watch?v=hpKhjKrBn9s&annotation_id=annotation_736168&feature=iv
-Atoms are electrically neutral (the number of protons equals the number of electrons)
- Two different models can be used to describe electron configuration
-Energy Level Model
-Bohr Model
- electrons occupy shells which are divided into orbitals
The Bohr diagrams places the number of neutrons and protons in the center and electrons in energy rings around the outside. Each energy ring has a maximum number it can hold
Learned in class:
Ring 1 – 2 electrons
Ring 2 – 8 electrons (octet)
Ring 3 – 8 electrons (octet)
Ring 4 – 18 electrons
----------------------------------------------------------
Ring 5 – 18 electrons
Ring 6 – 32 electrons
Ring 7 – 32 electrons
The electrons in the outer most energy ring are called valence electrons. These electrons are very important because they are involved in chemical reactions. They can be lost or gained so that the element will have 8 electrons in the outer most ring and become stable. The noble gases already have 8 electrons in their outer ring so they are stable and do not react readily
Now its your turn to try!
Atom Structure:
http://www.youtube.com/watch?v=hpKhjKrBn9s&annotation_id=annotation_736168&feature=iv
Bohr Diagrams:
http://www.youtube.com/watch?v=hpKhjKrBn9s&annotation_id=annotation_736168&feature=iv
Here are some videos on youtube that deal with the Atomic Structure and give a detailed explanation on Bohr Diagrams. Enjoy!
-Post by Ivy Gloria. Picture by Ren Flores
ELECTRONIC STRUCTURE: DRAWING ELECTRON DOT DIAGRAMS: November 1st, 2010
Drawing electron dot diagrams:
An atom of Neon can be represented by the diagram on the left. But in this case, we are drawing electronic dot diagrams. For neon, we must determine a) number of valence electrions b) place dots around the element to represent the valence electrons. Since there are 10 electrons, 2 go in the first shell but the rest (8 electrons) are in the second shell, therefore, these electrons are considered to be in the valence shell, and we label them.
- The nucleus is represented by the atomic symbol
- For individual elements determine the number of valence electrons (electrons in the outermost energy level of an atom; for most atoms, it is available to be gained, lost, or shared in the formation of chemical bonds)
- Electrons are represented by dots around the symbol
- Four orbitals (one of each side of the nucleus) each holding a max of 2 electrons
- Each orbital gets 1 electron before they pair up to make a lone pair (a pair of electrons- 2 of them)
An atom of Neon can be represented by the diagram on the left. But in this case, we are drawing electronic dot diagrams. For neon, we must determine a) number of valence electrions b) place dots around the element to represent the valence electrons. Since there are 10 electrons, 2 go in the first shell but the rest (8 electrons) are in the second shell, therefore, these electrons are considered to be in the valence shell, and we label them.
Lewis Diagrams for compounds and Ions:
-In covalent compounds electrons are shared
1. Determine the number of valence electrons for each atom in the molecule
2. Place atoms so that valence electrons are shared to fill each orbital.
Compound:
We have just learned how to draw a Lewis dot diagram for a single element and a compound. An Ionic Compound presented as an electronic dot diagram has the follow:
-An ionic compounds electrons transfer from one element to another
-Determine the number of valence electrons on the cation (+) and move these to the anion (-).
-Draw [ ] around the metal and non-metal (write the charges on the outside bracket)
 |
| An example of an ionic compound: Lewis Dot Diagram |
Next we have a more complicated diagram, because it consists of a "DOUBLE BOND".
DOUBLE & TRIPLE BONDS:
Sometimes the only way covalent compounds can fill all their valence levels is if they share more than one electron.
This periodic table can also help you when drawing Electronic Dot Diagrams. Notice a trend in each group (group 1 has 1 valence electron, group 15 has 5 valence electrons and so on)
Yay, you've mastered the art of drawing Electronic dot Diagrams!
Post by Ren Flores
28.10.10
TRENDS ON THE PERIODIC TABLE: October 28, 2010
Elements close to eachother on the periodic table display similar characteristics
There are 7 important periodic trends:
1) Reactivity2) Ion Charge
3) Melting Point
4) Atomic Radius5) Ionization Energy
6) Electronegativity
7) Density
1) REACTIVITY
- metals and non-metals shoe different trends
- the most reactive metal is Francium; the most reactive non-metal is Fluorine
-reactivity increases as you go down for metals and up for non-metals-Noble gases are very unreactive
2) ION CHARGES
- elements ion charges depend on their group (column)
3) MELTING POINT
- elements in the centre of the table have the highest melting point
- noble gases have the lowest melting points
- starting from the left and moving right, melting point increases (until the middle of the table, it then starts to decrease)
-an exception to this rule is Carbon. Carbon has a high melting point!
4) ATOMIC RADIUS
- radius decrease to the up and the right
- Helium has the smallest atomic radius
- Francium has the largest atomic radius
5) IONIZATION ENERGY- ionization energy is the energy needed to completely remove an electron from an atom
- it increases going up and to the right
- all noble gases have high ionization energy- Helium has the highest I.E.
- Francium has the lowest I.E.
- opposite trend from atomic radius
-Quick note: think about why Ionization energy has the opposite trend of the Atomic radius. Since the atomic radius gets smaller when ionization energy increases, it tells us that when the shells are smaller, the energy needed to completely remove an electron is easier, therefore, electrons can leave their small atomic radius which is an increase in ionization energy.
-Quick note: think about why Ionization energy has the opposite trend of the Atomic radius. Since the atomic radius gets smaller when ionization energy increases, it tells us that when the shells are smaller, the energy needed to completely remove an electron is easier, therefore, electrons can leave their small atomic radius which is an increase in ionization energy.
6) ELECTRONEGATIVITY
- refers to how much atoms want to gain electrons- same trend as I.E.
7) DENSITY
...yet to be learned!
and our own pictures!
Post by: Adrienne Ross (with pictures from Ren Flores)
26.10.10
ISOTOPES AND ATOMS: October 26, 2010
Today we learned about Isotopes! We have already learned that ions are atoms that are either missing or have extra electrons. Let's say an atom is missing a neutron or has an extra neutron. That type of atom is called an isotope. An atom is still the same element if it is missing an electron. The same goes for isotopes. They are still the same element. They are just a little different from every other atom of the same element.
*Note: the most common ion charge is listed on top (if theres 2 or more charges)
* Atomic mass - Atomic number = # of neutrons
*If you are given the atomic number and number of neutrons, add them to get the mass number
A decimal found in the atomic mass is an average of the isotopes
Going good so far? Great.
Mass spectrometry (MS) is an analytical technique that measures the mass-to-charge ratio of charged particles.[1] It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and other chemical compounds. The MS principle consists of ionizing chemical compounds to generate charged molecules or molecule fragments and measurement of their mass-to-charge ratios.[1] In a typical MS procedure:
- A sample is loaded onto the MS instrument, and undergoes vaporization
- The components of the sample are ionized by one of a variety of methods (e.g., by impacting them with an electron beam), which results in the formation of charged particles (ions)
- The ions are separated according to their mass-to-charge ratio in an analyzer by electromagnetic fields
- The ions are detected, usually by a quantitative method
- The ion signal is processed into mass spectra
Now its your turn! Fill out the chart on isotopes!
Answers: Row 1: 28 neutrons. Row 2: Mn(Manganese) Protons:25 Netruons: 31 Row 3: Mass # 12, Atomic #:6, # of protons: 6, # of neutrons 6
Post by Ren Flores
Subscribe to:
Posts (Atom)








































