Few points before we begin:
- Carbon chains can form 2 types of closed loops
- Alicyclics are loops usually made with single bonds
- If the parent chain is a loop standard naming rules apply with one addition: "cyclo" is added infront of the parent chain
There are 3 different ways to draw organic compounds:
- Complete structural diagram
- Condensed structural diagram *
- Line Diagram * (mostly used because its way easier to draw and you dont have to include all the hydrogens)
*Numbering can start anywhere and go clockwise or counterclockwise on the loop but side chain numbers MUST be the lowest possible!
So let us draw some to show you!
AN EXAMPLE of a condensed structural diagram
- A condensed structural diagram always shows carbons and hydrogens in it
Example of a line diagram:
- loops can also be side chains
- same rules apply but the side chain is given a cyclo- prefix
You also must know how to name a compound!
First, take note of what you see
- You see 2 lines connecting CH2 and C, so you know this organic compound forms a double bond and you must use the ending -ene
- You count the longest carbon chain to be 5 so we use -pent
- -pent + -ene = pentene and this bond occurs at 1 so: 1 pentene
- we see a loop on #3 of the carbon chain so we must use to prefix -CYCLO and there are 3 of the CH's on it so we use propyl and carbon and hydrogen on #2
- #2: 2 methyl
- #3: 3 cyclopropyl
Together this compound is called:
2 methyl 3 cyclopropyl 1 pentene
AROMATICS:
2ND PART OF TODAYS CLASS
- Benzene (C6H6) is a cyclic hydrocarbon with unique bonds between the carbon atoms
- Structurally it can be drawn with alternating double bonds
- Careful analysis shows that all 6 C-C bonds are identical and really represent 1.5 bond
- This is due to electron resonance for electrons are free to move all around the ring
AROMATIC NOMENCLATURE:
- A benzene molecule is given a special diagram to show its unique bond structure
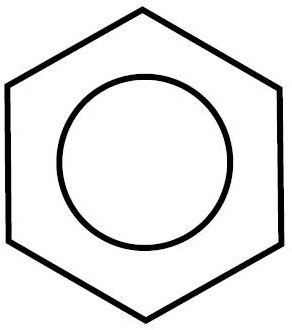
- A benzene can be a parent chain or a side chain
- As a side chain it is given the name PHENYL*
Draw the line diagrams of the following:
a) 1, 4 diethyl 2 methyl Benzene
b) 1,3,5 triethyl 2,4,6 tripropyl benzene
c) 1, 3 diethyl 5 propyl benzene
and that is it!
Post by Ren Ren Flores








No comments:
Post a Comment